Understanding electric vehicle (EV) technology has become increasingly important as these vehicles gain market share worldwide. Behind every EV lies a complex battery system that not only determines the vehicle’s performance but also impacts its safety profile. With recent news reports of EV-related incidents, including battery fires, gaining a deeper understanding of battery technologies has become essential for consumers, manufacturers, and safety regulators alike. The chemistry, structure, and thermal management of these batteries directly influence how vehicles perform and how they might respond in critical situations.
A few weeks ago, I was on a business trip and got an unexpected upgrade at the rental car counter. The agent was nice enough to lend me a brand-new electric vehicle instead of my usual gas-powered sedan. The best part? I didn’t have to refill the tank (like I always did) before returning it—or even recharge it—with one catch: the total mileage needed to stay under 200 miles. The car was quiet and smooth, and I was genuinely happy with the experience. As someone with an engineering background, I found myself wondering what kind of batteries were powering this vehicle and why the a specific 200-mile limit. This exploration of EV battery technology aims to answer these questions.
The Dominance of NMC and LFP Technologies
While various battery chemistries exist in the electric vehicle space, two technologies have emerged as clear market leaders: Lithium Nickel, Manganese Cobalt Oxide (NMC) and Lithium Iron Phosphate (LFP). Together, these powerhouses dominate approximately 85% of the current EV market, with NMC holding roughly 55% of the global market share and LFP accounting for about 30%.
Each chemistry offers distinct advantages and limitations that make it suitable for different applications within the EV ecosystem. Understanding these differences provides insight into why manufacturers choose specific battery technologies for particular vehicle models and use cases.
Battery Cell Structure and Components
Both NMC and LFP batteries are lithium-ion technologies that share similar architectural principles while differing significantly in their material composition. A single battery cell contains several key components that work together to store and release energy.
The cathode (positive electrode) represents the defining difference between these battery types. NMC batteries utilize Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO₂) with various ratios of nickel, manganese, and cobalt. Common formulations include NMC111 (equal parts), NMC532, and NMC811, each offering different performance characteristics. LFP batteries, by contrast, employ Lithium Iron Phosphate (LiFePO₄) as the active cathode material.
The anode (negative electrode) is typically graphite in both chemistries, though silicon-graphite composites are increasingly appearing in newer high-energy-density designs. When charged, lithium ions are stored within the layered structure of the graphite.
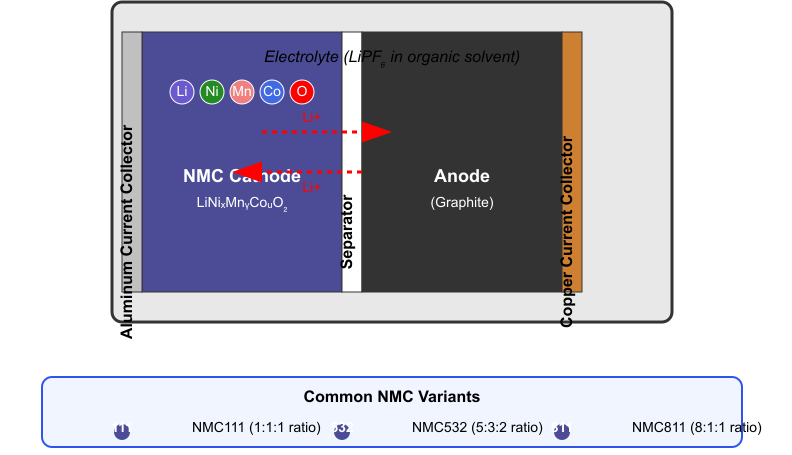
Between these electrodes sits an electrolyte—a lithium salt solution in organic solvents—that enables the movement of lithium ions. A separator prevents direct contact between the electrodes while allowing ion transport. The entire assembly is contained within a casing that provides structural integrity and protection.
As shown in the diagram, the NMC battery structure features the cathode (left side, blue) made of lithium, nickel, manganese, and cobalt elements, separated from the graphite anode (right side, black) by a separator. The red dotted arrows illustrate the movement of lithium ions through the electrolyte during charging and discharging cycles. The diagram also shows the current collectors (aluminum for the cathode and copper for the anode) that facilitate the flow of electrons in the external circuit.
Physical Construction and Casing Types
The physical form of battery cells significantly influences their performance, thermal management, and integration into vehicle systems. Three primary formats dominate the industry:
Cylindrical cells resemble oversized versions of household batteries, with metal casings (typically steel or aluminum) that provide excellent structural integrity and internal pressure containment. Diameters range from 18mm to 46mm, with various heights. Some early electric vehicles employed thousands of cylindrical cells in their battery packs, and many manufacturers continue to use this format, particularly with NMC chemistry.
Prismatic cells feature rigid rectangular aluminum casings that offer efficient space utilization. These larger cells require fewer electrical connections in a complete battery pack but need more sophisticated cooling solutions. LFP chemistry predominantly appears in prismatic formats, though NMC variants also utilize this form factor.
Pouch cells use flexible aluminum-polymer laminate enclosures that reduce weight but require external structural support. The large surface area facilitates cooling, but these cells need careful management of expansion during cycling. Pouch formats are more commonly paired with NMC chemistry.
The casing doesn’t merely contain the active components—it plays critical roles in heat management, pressure regulation, and protection from environmental factors. Material choice and design significantly impact cell performance, safety, and longevity.
The Electrochemical Dance: How Lithium-Ion Batteries Work
The fundamental operating principle of both battery types involves the movement of lithium ions between the electrodes, accompanied by electron flow through an external circuit. This electron flow provides the electrical power that drives the vehicle.
During discharge (when powering the vehicle), lithium ions move from the anode to the cathode through the electrolyte. Simultaneously, electrons travel from the anode to the cathode through the external circuit, powering the electric motor. During charging, the process reverses, with an external power source forcing electrons and lithium ions back to the anode, where they’re stored until needed.
This intercalation mechanism—where lithium ions are inserted into and extracted from the electrode materials without significant structural changes—enables the thousands of cycles expected from modern EV batteries.
The diagram clearly illustrates this process for an NMC battery, showing how the lithium ions (represented by the Li symbol in a purple circle) move through the separator when the battery is in use. The different elemental components of the NMC cathode (Ni, Mn, Co, and O) are also displayed, highlighting the complex composition that gives this battery type its unique properties.
Performance Characteristics: NMC vs. LFP
Energy Density
Energy density—the amount of energy stored per unit weight or volume—represents perhaps the most significant difference between these technologies:
NMC batteries achieve 200-350 Wh/kg and 550-750 Wh/L, allowing for longer driving ranges within space and weight constraints. Premium EVs with NMC batteries routinely achieve ranges exceeding 300 miles.
LFP batteries deliver 90-160 Wh/kg and 350-450 Wh/L. This lower energy density explains the 200-mile limit on my rental car—it likely used an LFP battery optimized for urban and regional use rather than long-distance travel. The pack will also be approximately 20% heavier than an equivalent-capacity NMC system.
Operating Voltage
The nominal operating voltage differs between these chemistries, contributing to the energy density gap:
- NMC cells operate at 3.6-3.7V nominal voltage
- LFP cells operate at approximately 3.2V nominal voltage
This voltage difference means more LFP cells must be connected in series to achieve the same pack voltage, impacting system design and electronics.
Cycle Life and Longevity
LFP batteries demonstrate superior cycle life, typically achieving 3,000-6,000 full equivalent cycles before reaching 80% of their original capacity. This exceptional longevity stems from the inherent stability of the olivine phosphate structure, which maintains integrity through numerous lithiation/delithiation cycles.
NMC batteries generally achieve 1,000-2,000 full equivalent cycles before reaching the same capacity threshold. Higher nickel content in advanced NMC formulations can further reduce cycle life while boosting energy density, representing a critical engineering trade-off. As shown in the diagram’s lower section, NMC variants range from NMC111 (equal parts nickel, manganese, and cobalt) to NMC811 (8:1:1 ratio), with each variation offering different performance characteristics.
Both technologies experience degradation through similar mechanisms:
- Growth of the solid-electrolyte interphase (SEI) layer, consuming lithium ions and increasing internal resistance
- Structural changes in active materials during cycling
- Lithium plating during fast charging or low-temperature operation
The average capacity degradation rate for modern EV batteries ranges from 1.8% to 2.3% annually, allowing for long service lives. Most manufacturers offer warranties covering 8-10 years or 100,000-150,000 miles.
Thermal Characteristics and Safety
Thermal stability represents another key differentiator between these technologies. LFP batteries offer exceptional thermal stability, with onset of thermal runaway typically occurring above 270°C. This inherent safety margin often allows for simpler thermal management systems.
NMC batteries demonstrate lower thermal stability thresholds, with runaway typically initiating between 150-200°C depending on the specific formulation. Higher nickel content generally correlates with reduced thermal stability. This characteristic necessitates more aggressive cooling systems and robust safety measures.
Temperature Performance
Environmental operating conditions significantly impact battery performance:
NMC batteries typically maintain reasonable performance down to -20°C, with gradual capacity reduction as temperatures drop. They perform well in moderate heat but require thermal management above 45°C.
LFP batteries show more pronounced capacity and power loss at low temperatures, with significant performance degradation below -10°C. However, they often demonstrate better calendar aging characteristics at elevated temperatures.
This temperature sensitivity explains why vehicle range can vary significantly based on climate and season, sometimes by 20-30% between optimal and extreme conditions.
Battery Management Systems
The distinct characteristics of each chemistry necessitate specialized approaches to battery management. NMC batteries feature sloped voltage curves that facilitate state-of-charge estimation through voltage measurement, while LFP batteries exhibit extremely flat voltage profiles through much of their operating range, making accurate state-of-charge determination challenging.
Thermal management also differs substantially. NMC packs typically require active liquid cooling systems due to higher heat generation and lower thermal stability thresholds. Some LFP applications can function effectively with passive or air cooling systems, potentially reducing system complexity and cost.
Raw Materials and Sustainability
LFP batteries utilize iron and phosphate, both abundant and widely available materials with established supply chains. The absence of cobalt and nickel addresses sustainability concerns related to resource scarcity and mining practices.
NMC batteries require nickel and cobalt, both facing supply constraints and ethical sourcing challenges. Industry trends show a progressive reduction in cobalt content through successive NMC formulations (from NMC111 to NMC811 and beyond), partially addressing these concerns while maintaining performance.
Conclusion
The technical differentiation between NMC and LFP batteries exemplifies the engineering trade-offs inherent in electric vehicle design. NMC offers superior energy density at the expense of cycle life, thermal stability, and material sustainability. LFP provides exceptional longevity and safety with abundant raw materials but at lower energy density.
Understanding these trade-offs helps explain design decisions in modern electric vehicles. My rental car’s 200-mile range limitation likely stemmed from the fundamental energy density constraints of LFP technology—a rational engineering choice for a vehicle intended primarily for urban and regional use.
As battery technology continues to evolve, we can expect both chemistries to improve across multiple performance metrics. NMC formulations will likely continue reducing cobalt content while incorporating advanced anodes to boost energy density. LFP development will focus on improving energy density and low-temperature performance through material engineering and cell design optimization.
These advancements will further accelerate the electric vehicle revolution, making sustainable transportation increasingly practical, affordable, and compelling across diverse applications and use cases.
Any questions about EV battery technology or a claim involving EVs? Contact us today for expert opinion.



